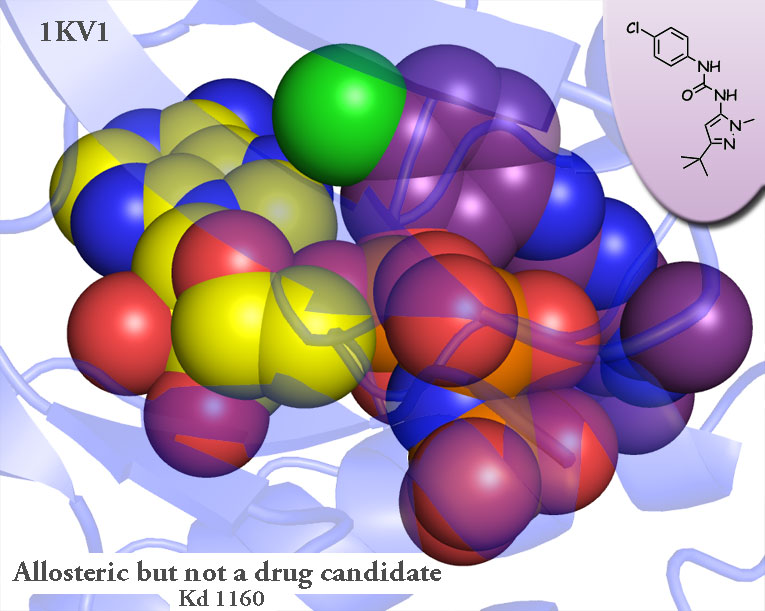
Allosterism: A Diatribe
Posted by kinasepro on May 25, 2009
So just what is an allosteric kinase inhibitor?
Google’s I’m feelin lucky hit ?
(āl’ō-stěr’ĭz’əm)
A change in the activity and conformation of an enzyme resulting from the binding of a compound at a site on the enzyme other than the active binding site.
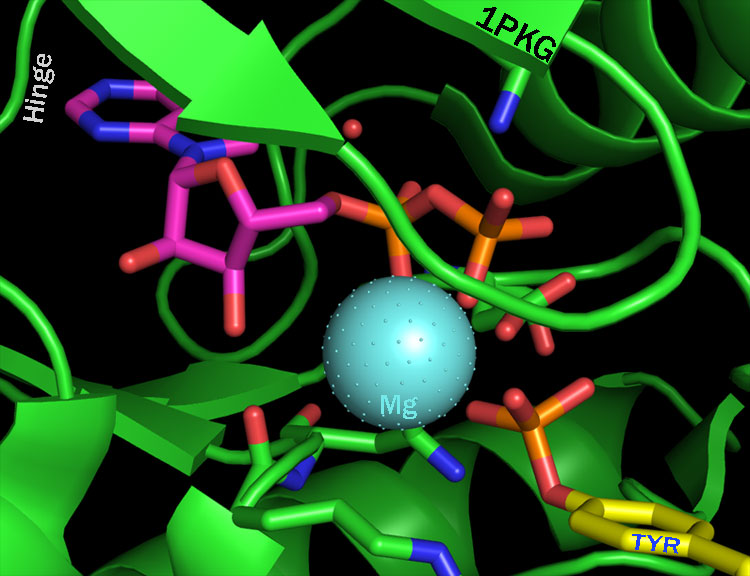
Ok, so what is an ‘active binding site’ of a kinase? Universally this is held as the region to which ATP binds to the enzyme and a site which itself is structurally analogous amongst the serine / threonine, tyrosine, phosphoinisotide kinases, etc. To further our understanding lets turn the PDB. 1PKG is a beautiful example of the kinase domain of c-kit bound to ADP in a state that appears to be just after it transferred a phoshpate to a substrate tyrosine residue (a residue also of c-kit, its an auto-phosphorylation reaction) As inadequate as any crystal structure may be at describing the underlying reality this one give us an exquisite glimpse of the catalytic reality that is phosphorylation.
A ‘steric’ inhibitor of this process must therefore physically occupy the space that would otherwise normally be occupied by the participants of this reaction. As stated and by convention this is nearly always to consider the occupation of the nucleoside binding region by some broadly defined organic moiety, but there really is no reason a steric inhibitor could not also block the substrate binding region. It appears to be a bit more difficult to find molecules willing to do this sort of thing.
So how about Gleevec? Well the setup poll for this post shows an interesting split in responses. 24/62/14 % in the yes no maybe camps respectively at the time if this writing [a mere 264 respondents in case you were wondering]. Interesting because the ATP binding site thus defined leads to the unavoidable conclusion that Gleevec satisfies the condition of a molecule which when bound to a kinase will spatially (sterically) precludes the binding of ATP in a catalytically active form.
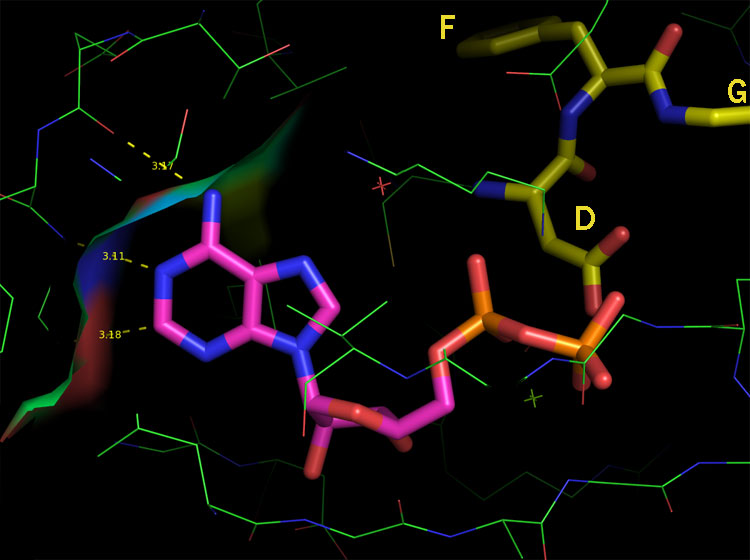
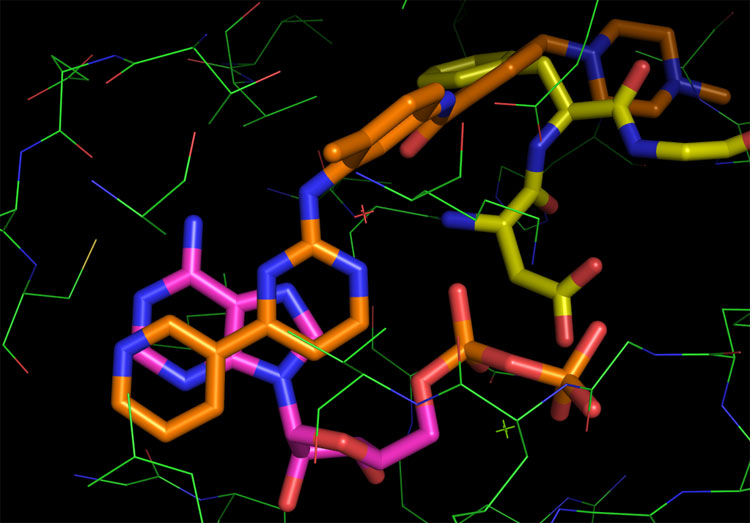
To continue with our c-Kit example, this is something 1T46 illustrates quite nicely. The picture below is of Gleevec in its bound conformation from 1T46 overlayed with ADP from 1PKG. Clearly the pyridine and part of the pyrimidine rings occupy this space:
This image makes it so blindingly obviously that Gleevec is a Steric inhibitor that I find it a challenge to put an intelligible string of words together in such a way to state this fact nearly as succinctly as can be seen here. What’s more everybody knows this. There’s really nothing new in any of the above images or statements and the general idea has been common knowledge for the passed 7 to 10 years depending on where you work. Clearly the Craik Lab knows this stuff, Right? So the only question for me is:
Why does something like this make a recent Science article:
The discussion alluded to focuses on that Gleevec and nilotinib do something that dasatinib does not as evidenced by 1H NMR and crystallography (incidentally there are other ways of doing more or less the same thing). This behavior is not in question as the A-loop is simply not tied down by dasatinib and is free to waft around and be phosphorylated bound or unbound. To consider the DFG effect an allosteric one is fine indeed, but the drugs that have emerged by binding in the region are not allosteric. If this is not clear to you, then I suggest staring at the images above for a few more minutes.
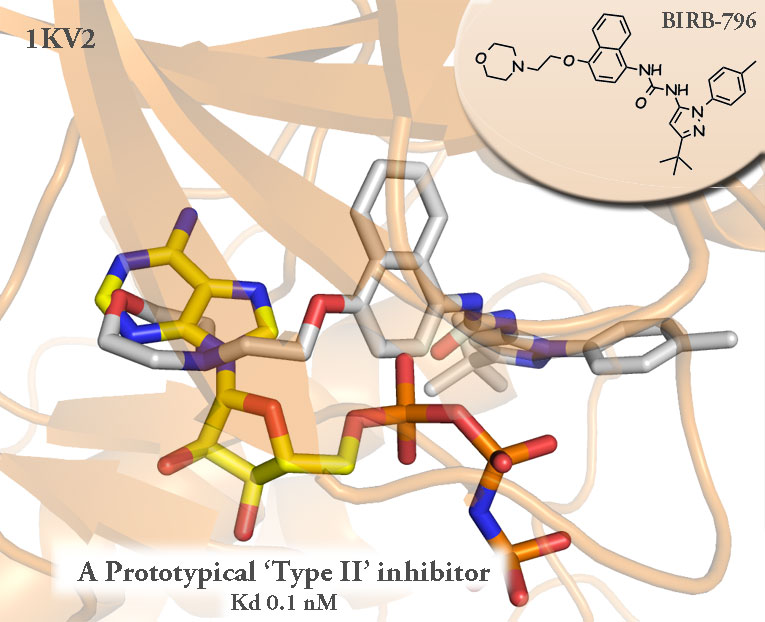
Much of the confusion likely harkens back to what seems to be a general misunderstanding of the original BIRB-796 ‘allosteric’ paper. That paper showed the uM inhibition of P38 by a purely allosteric inhibitor. It’s possible, but to approach physiologically relevant and disease modifying kinds of potency you have to tether it to something Steric. It has to hit the hinge. To date all of the so called ‘Type II‘ inhibitors must sterically occlude the binding of ATP in order to find their way down to medicinally useful concentrations.
(AMP overlayed in yellow where ATP normally binds to P38 in the following images.)
Even the authors of this paper note when speaking on an 11-fold improvement in biochemical activity from the addition of the morpholine-ethoxy moeitey: “This hydrogen bond, equivalent to the one made by the N1 atom of the adenine base of ATP… establishing interactions in the ATP pocket… ‘
And yet the myth lives on that BIRB-796, sorafenib, and imatinib are somehow allosteric inhibitors? If, as I’ve now shown, BIRB is not an allosteric inhibitor then where does that leave Gleevec? Un-tether imatinib from the ATP binding region and I don’t believe anyone can show meaningful activity to a kinase. Gleevec is simply not an “allosterically active drug”.
Got a compound that binds in this region and doesn’t sterically occlude ATP? Show me a drug candidate and I’ll happily eat my words! Till then can you all please stop calling these compounds allosteric?


ATP->ADP said
Hi KP, Nice writeup! I think the word allosteric is a very loosely used one. The % change in the inhibition of a true allosteric inhibitor should not change no matter what ATP conc. you use in the biochemical assay. I’m sure all type II inhibitors are indirectly ATP competitive (make what you will of that!;-)) as they sterically occlude the binding of ATP (DFG in “out” position clashes with phosphate groups). Only when there is a co-binding with ATP as seen in certain MAP kinases can we call the inhibitor allosteric. Or as you brought it up sometime earlier, a Chk1 inhibitor from Merck (https://kinasepro.wordpress.com/2009/01/20/whats-that-doin-there/) can qualify as an allosteric inhibitor. But the fact is that drug discovery is so interdisciplinary and in the end it hardly matters what one calls an inhibitor!
elspud said
Amen brother. I get accused of being fixated on this point…good to know I’m not alone.
weirdo said
The word “allosteric” is certainly used loosely, but it not defined loosely. KP’s point is clearly valid: Type II inhibitors are not allosteric inhibitors. Some would add “by the classic definition”, but then I would suggest a new term (preferably something with “omic” in it).
milkshake said
when “type II” and “allosteric” enter meeting-speak as a catchphrase for the project managers to impress their non-chemist superiors, the terms became emptied of their original meaning.
See how word “chiral” (as of “a chiral building block”) morphed in the meeting-speak from the original meaning “chirality-possessing” into “optically pure”. When I got annoyed once and pointed out that racemic compounds are all chiral, too, they all looked up as if antennae were sprouting out from my forehead)
viledonkey said
I’m with Elspud, glad to see I’m not alone!
IMHO, if a compound ‘plays’ with the ATP pocket, albeit in a minor sense, then it isn’t an true allosteric inhibitor. Compounds that cause inhibition of kinases by binding with the enzyme at some other distal site away from the ATP-pocket, then we are dealing with an allosteric inhibitor. The literature does have them, but they are not yet all mainstream: MEK, Akt PH-domain, JNK JIP-1, Plk polo-box domain just to name a few. I know we are entering the realm of protein-protein interactions……but it is a direction we may need to head in!
M. Blue said
KP,
Good write up indeed, as always. However, the appropriate term is “orthosteric” rather than just “steric”. Correct?? May be I’m just nitpicking!! :))
Wavefunction said
Great write up. Should be a fitting reminder to those who think ATP-competitive inhibitors are also “allosteric” because they happen to affect the DFG loop or substrate or a combination thereof. It’s time somebody took a stand against all this confusion. Plus in my opinion, “true” allosteric inhibitors should not have as many cross-reactivity issues; that’s supposed to be the purpose of allosteric kinase inhibition in the first place, a purpose not served by many of these so-called allosteric compounds.
szacsa said
I agree nice and clear writeup. I found something interesting in National Institutes of Health Chemical Genomics Center’s Assay Guidance Manual. Authors stated that an allosteric compound, which act on another site but active site, can showed competitive kinetics. (Referred to Segal’s Enzyme Kinetics book)
http://www.ncgc.nih.gov/guidance/section12.html#allosteric-inhibition
I don’t know if this type of inhibition mechanism exits in the field of kinases.