…the Bee Gee’s and R406 have in common?
R-788 (a prodrug of R-406) has shown promise in a Ph2b trial. Partner Plz?
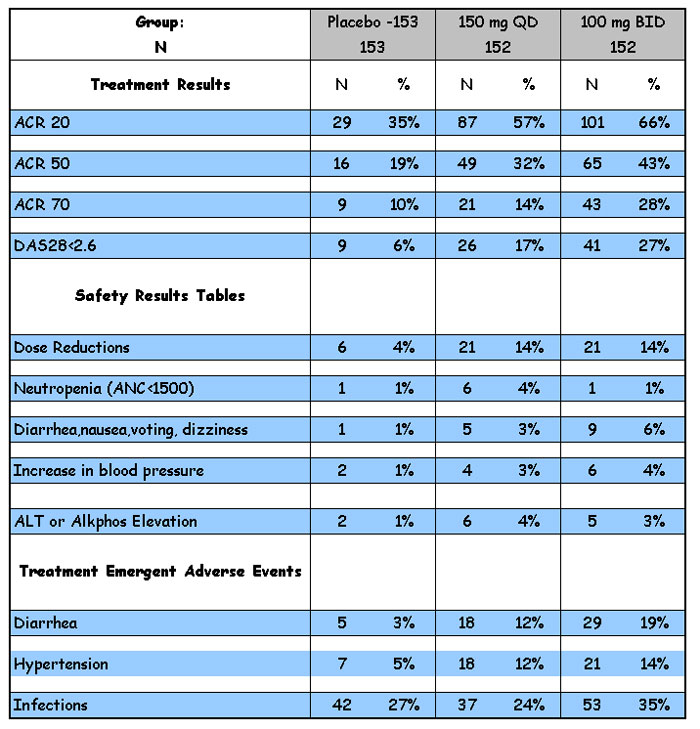 still no BMCL
still no BMCL
Posted by kinasepro on July 10, 2009
…the Bee Gee’s and R406 have in common?
R-788 (a prodrug of R-406) has shown promise in a Ph2b trial. Partner Plz?
 still no BMCL
still no BMCL
Posted in Rigel, SYK | 2 Comments »
Posted by kinasepro on April 9, 2009
The saga continues… So there’s some Shareholders alleging inappropriate behavior. The short of their argument goes something like:
Dec 13, 07: Rigel: R788 is great!

Feb 6, 08: Rigel sells 5,000,000 shares at $27
Oct 27, 08 Rigel presents full study details:
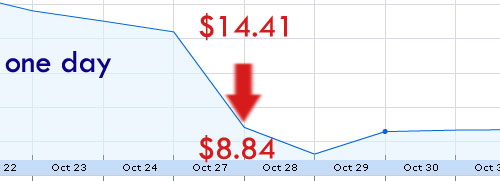
And now there’s a lawsuit presumably claiming that Rigel didn’t share material data during the December 13 releases and 8k filing that when said data did finally come out, it dropped the stock considerably…
Posted in Rigel, SYK | Leave a Comment »
Posted by kinasepro on December 7, 2007
Rigel gets 5M & some good news from Pfizer:
…Pfizer (NYSE: PFE), has begun a Phase 1 clinical trial of an inhaled formulation of Rigel’s small molecule syk kinase inhibitor, R343, for the treatment of allergic asthma…
Posted in Pfizer, Rigel, SYK | 2 Comments »
Posted by kinasepro on November 9, 2007
R-788 is Rigel’s SYK / Flt3 inhibiting prodrug of R-406, and despite a positive sounding press release on their Ph2 ITP study, the market is reacting negatively so far by knocking 20% off of the stock price.
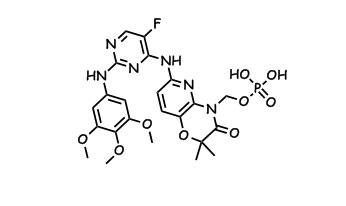
the name suggests disodium.
Posted in Flt3, Rigel, SYK | 15 Comments »
Posted by kinasepro on November 6, 2006
Hello Novartis, I see the title of your recent offering is ‘blablabla as inhibitors of Zap70 and / or Syk Kinase.’ The discerning Kinasefan will recognize at least 1/2 of the following molecule which is incidentally makes the highlight reel as example 1. Sure it looks the like the Rigel stuff, but thats really just the part of the game eh? (you guessed it, the 4-halo pyrimidyl’s are part of the table)

Looking passed the abstract one can learn that yah, sure, some of these examples are going to have some SYK activity (100nM – 10 uM), but primary target is clearly Zap70 and compounds are also tested against ALK.

I see in the experimental you guys made 1.2 grams of compound 140, and reported the ic50 of 10nM for the compound. I’m not sure why, I mean I’m not entirely sure why you’d drop the dime on that, but clearly it was a lead compound.
CNC1=CC(NC2=NC(NC3=CC=C(OCCO4)C4=C3S(=O)(N)=O)=CC=N2)=CC=C1C CNC1=CC(NC2=NC(NC3=CC=C(OCCO4)C4=C3S(=O)(N)=O)=CC=N2)=CC=C1C
Posted in Novartis, SYK, Zap70 | Leave a Comment »
Posted by kinasepro on October 21, 2006
Hey Rigel, SYK prodrugs eh? I see you guys have been on this stuff like white on rice for a couple years. I suppose Kinasepro could wait for the Biorg. Med. Chem. Lett. to come out some time in 2010, but I’m gonna see if I couldn’t kick up a first draft to help you guys out. Here Goes:
So R112 sucks but since we’ve got this really cool assay and Pfizer’s had a bag of cash dangling out there for us, its not like we were just going to give up on the target. Yah in case you were wondering we all lost a ton of money back then.
Anyhow the early SAR for this project was carved around the arylaminopyrimidine scaffold based on a library hit, and and we put the kitchen sink both meta and para on the pyrimidine (WO2003063794 – 7/8/03; WO2004014382 – 2/19/04).

R112 had crummy solubility, so we thought the idea of shoving it up peoples noses sounded pretty good. You know, like an allergy indication – something sort of topical where you might get away without solubility? Put it together with some of the finest, and most advanced dispersal agents known to mankind and throw it into a few hundred sneezing folks and lets see what happens. Somehow it didn’t work out. Go figure, thats just how these things go sometimes I guess.But as I was saying, some of our best early compounds were bicycles(1), and since I had some luck with indoles on a previous project I really put a beat down on that indazole, and indole chemistry. C’mon they’re supposed to be phenol bioisosteres. It just wasn’t working out until we started making benzoxazines (2) and then we knew: these comopounds are for real!
That brings me to these second generation analogs where we got a feel for the SAR. Along the road we learned a few things: Namely as we honed in on the benzoxazines we found that the saturated ring preferred a hindered center next to oxygen (4), that the other aryl was more or less solvent exposed, and that we could get away with putting tons of stuff out there (3). Oh yah – and that an azabenzoxazine improved our drug like properties (5). Sure they were harder to make, but you gotta do what you gotta do, eh? The pyrimidine wouldn’t budge, but oh yah, we did find some success with a few non-benzoxazines (WO2005013996). We had a handful of non-benzoxazines, but they just didn’t cut it. Insoluble as it is R406 was the best of the lot.
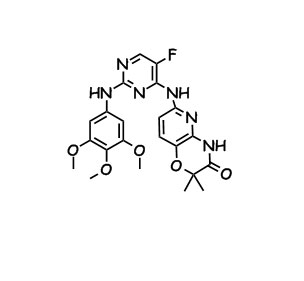 We nominated the benzenesulfonic acid salt, and the phase 1 study was done around the same time as our Phase II for R112. This compound is oral and we proved we were hitting a biomarker but for some reason the stock didn’t go back up =<
We nominated the benzenesulfonic acid salt, and the phase 1 study was done around the same time as our Phase II for R112. This compound is oral and we proved we were hitting a biomarker but for some reason the stock didn’t go back up =<
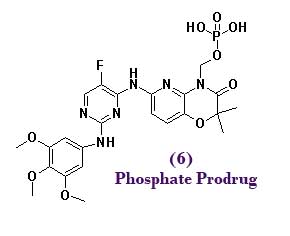 To polish off this project we looked at some phosphonate prodrugs which eventually led to R788 (6), but pretty much everybody is on HCV now. US20060234983 published Thursday, and WO2006078846 came out back in January – they’re all a part of this work. The non-prodrug aza-analog of R406 had a solubility of like 1 ug / mL – while the phosphonate is like *POW* 5 mg/mL, it also gives us us a %f of about 30%. Oh, and R788 rox k thx.** The above is merely a lighthearted satirical dramatization derived from Kinasepro’s keen ability to filter the patent literature and press releases. Please feel free to complain in the comments or to kinasepro@gmail.com
To polish off this project we looked at some phosphonate prodrugs which eventually led to R788 (6), but pretty much everybody is on HCV now. US20060234983 published Thursday, and WO2006078846 came out back in January – they’re all a part of this work. The non-prodrug aza-analog of R406 had a solubility of like 1 ug / mL – while the phosphonate is like *POW* 5 mg/mL, it also gives us us a %f of about 30%. Oh, and R788 rox k thx.** The above is merely a lighthearted satirical dramatization derived from Kinasepro’s keen ability to filter the patent literature and press releases. Please feel free to complain in the comments or to kinasepro@gmail.com
>> Updated 10/23 >> Fixed a couple chemdraw errors, and would like to add a reader submission link to an article on R406 from August ’06 (free link). They got an xray but the picture… well the picture leaves something to be desired.
Posted in Rigel, SYK | 4 Comments »